Abstract
Background: Sapacitabine is a novel, orally bioavailable, cytosine nucleoside analogue with a unique mechanism that generates single-stranded DNA breaks which are converted into double-stranded DNA breaks resulting in cell death. Previous studies of sapacitabine alone and alternating with decitabine (DAC) have demonstrated activity in acute myeloid leukemia (AML). Preclinical studies have suggested synergy with the combination of sapacitabine and a BCL-2 inhibitor. We conducted a study investigating the combination of sapacitabine and venetoclax (VEN) as an entirely oral regimen for patients with relapsed/refractory (R/R) AML.
Methods: This is a phase I/II study for patients ≥ 18 years with R/R AML or MDS with blasts ≥10% investigating the safety and efficacy of escalating doses of sapacitabine combined with VEN. Eligibility included ECOG performance status ≤ 2 and adequate organ function. Two cohorts were investigated: Cohort 1 studied sapacitabine given PO BID on D1-5; Cohort 2 studied sapacitabine given PO BID on D1-3 and D8-10 of each cycle, which was defined as 4 weeks. Sapacitabine was escalated from a starting dose of 250mg. VEN was given to a target dose of 400mg PO on D1-14 of each cycle. VEN ramp-up was utilized, starting at 100mg on D1, 200mg on D2, and 400mg on days 3 through 14. Adjustments to the VEN dose were made based on concomitant CYP3A inhibitors.
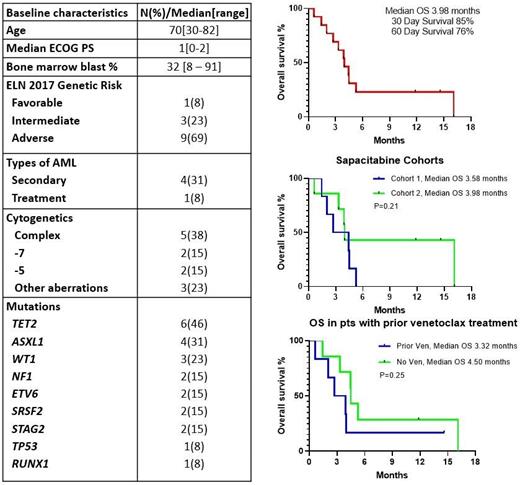
Results: Between 03/2020 and 03/2021, 13 pts were enrolled in the study. Baseline characteristics are shown in table 1. The median age was 70 years (range, 30-82) and 8 pts (62%) were aged ≥ 70 years. One pt (7%%) had favorable risk, 3 (23%) had intermediate risk, and 9 (70%) had adverse risk karyotype; 5 pts (38%) had a complex karyotype. The most common mutations detected by next-generation sequencing were: TET2 (46%), ASXL1 (30%), NRAS (30%), and WT1 (23%%). One patient (8%) had a TP53 mutation. This was a heavily pretreated population, having received a median of 2 prior therapies (1 - 9), including 6 patients (46%) having received prior intensive chemotherapy and 4 patients (30%) with prior allogeneic SCT. Six patients (46%) had received prior VEN-based therapy. Among 13 evaluable patients, there was 1 (8%) complete response with incomplete platelet recovery (CRp). Patients received a median of 1 cycle (1-9) on protocol. Five patients (38%) were able to remain on protocol without a protocol defined response, but with clinical benefit and good tolerability, for ≥2 cycles.
With a median follow up of 14.61 months (1-16), the median overall survival (OS) was 3.8 months with a 60-day survival rate of 76% (Figure 1). There was no significant difference in OS between the two dosing schedules. The median OS among patients that had and had not received prior VEN was 3.32 months and 4.5 months, respectively (P=0.25). The patient that achieved a CRi had secondary AML with 7q deletion, an ASXL1 mutation, and only single-agent DAC as prior therapy.
The oral chemotherapy combination was well tolerated, with 4- and 8-week mortality of 8% and 15%, respectively. The most common grade 3/4 non-hematologic adverse events regardless of attribution were: febrile neutropenia (31%), cellulitis (23%) pneumonia (15%), hypophosphatemia (8%), hypokalemia (8%). Two patients (15%) had grade 5 sepsis on study in the setting of active AML.
Conclusion: In a heavily pretreated population of patients with R/R AML, the combination of sapacitabine and VEN was well tolerated and feasible to be administered as a completely oral, outpatient regimen. Most patients had multiple prior cycles of nucleoside analogue containing regimens and the only notable responder had only prior DAC. Further study of this well-tolerated combination in less heavily pretreated patients may be considered.
Kantarjian: Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Aptitude Health: Honoraria; NOVA Research: Honoraria; Ascentage: Research Funding; Ipsen Pharmaceuticals: Honoraria; Astra Zeneca: Honoraria; Astellas Health: Honoraria; BMS: Research Funding; Daiichi-Sankyo: Research Funding; KAHR Medical Ltd: Honoraria; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. DiNardo: Novartis: Honoraria; Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Agios/Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Borthakur: GSK: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Protagonist: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; ArgenX: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; Ryvu: Research Funding. Daver: ImmunoGen: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Hanmi: Research Funding; Sevier: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Pemmaraju: Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Sager Strong Foundation: Other; Daiichi Sankyo, Inc.: Other, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy; Aptitude Health: Consultancy; Springer Science + Business Media: Other; Roche Diagnostics: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; LFB Biotechnologies: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; MustangBio: Consultancy, Other; Cellectis S.A. ADR: Other, Research Funding; Samus: Other, Research Funding; Plexxicon: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; CareDx, Inc.: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Alvarado: Sun Pharma: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Astex Pharmaceuticals: Research Funding; CytomX Therapeutics: Consultancy; BerGenBio: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; MEI Pharma: Research Funding. Burger: AstraZeneca: Consultancy; Beigene: Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Ravandi: Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding. Kadia: AstraZeneca: Other; Ascentage: Other; Jazz: Consultancy; BMS: Other: Grant/research support; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Amgen: Other: Grant/research support; Cure: Speakers Bureau; Dalichi Sankyo: Consultancy; Genfleet: Other; Cellonkos: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Novartis: Consultancy; Pfizer: Consultancy, Other; Liberum: Consultancy; Genentech: Consultancy, Other: Grant/research support; Astellas: Other.